Axsome Therapeutics, Inc. - Common Stock (AXSM)
183.30
+0.00 (0.00%)
NASDAQ · Last Trade: Feb 18th, 8:58 AM EST

The healthcare company is set to release its fourth-quarter results later this month.
Via The Motley Fool · February 5, 2026

Are we witnessing the birth of a biotech giant?
Via The Motley Fool · February 3, 2026
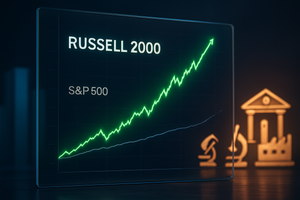
In a move that has rewritten the record books of Wall Street, the small-cap-heavy Russell 2000 index recently concluded a historic 15-session winning streak of daily outperformance against the S&P 500. This rally, which reached its zenith on January 22, 2026, represents the longest period of small-cap dominance since
Via MarketMinute · January 26, 2026
AXSOME THERAPEUTICS INC (NASDAQ:AXSM) Fits the Minervini Growth Momentum Strategychartmill.com
Via Chartmill · January 12, 2026

Piper Sandler kept an ‘Overweight’ rating on Axsome Therapeutics’ shares, and raised the price target to $223 from $148.
Via Stocktwits · January 16, 2026

Via MarketBeat · January 8, 2026

These could be the next biotech giants.
Via The Motley Fool · January 8, 2026
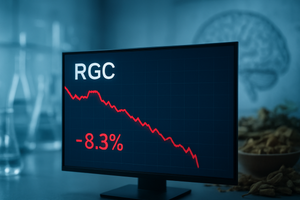
As the final bells rang on the 2025 trading year, investors in Regencell Bioscience (NASDAQ: RGC) were met with a sobering reality check. The Hong Kong-based biotech firm, which has enjoyed a meteoric and often volatile rise over the past twelve months, saw its stock slide 8.3% on December
Via MarketMinute · January 1, 2026
Here are the top movers in Wednesday's session.chartmill.com
Via Chartmill · December 31, 2025
Axsome Therapeutics shareholders got a New Year's gift from the FDA.
Via The Motley Fool · December 31, 2025
The market is filled with gapping stocks in Wednesday's session.chartmill.com
Via Chartmill · December 31, 2025

AXS-12 is being developed to treat cataplexy in narcolepsy by targeting norepinephrine and dopamine activity in the brain.
Via Stocktwits · December 31, 2025

As 2025 draws to a close, a powerful undercurrent of technological innovation, extending far beyond the pervasive influence of artificial intelligence, is demonstrably reshaping the global stock market. From the intricate defenses of cybersecurity to the vast frontiers of space technology, and from life-saving biotechnological breakthroughs to the efficiency gains
Via MarketMinute · December 11, 2025

These drugmakers are poised to profit from their innovative engines.
Via The Motley Fool · December 1, 2025

Axsome (AXSM) Q3 2025 Earnings Call Transcript
Via The Motley Fool · November 27, 2025

There might still be time to get in on the ground floor.
Via The Motley Fool · November 10, 2025

Axsome Therapeutics Q3 2025 results: Revenue beat expectations with strong product sales, but the company reported a wider-than-expected loss.
Via Chartmill · November 3, 2025

Via Benzinga · October 31, 2025

Both should have significant catalysts by the end of the decade.
Via The Motley Fool · September 30, 2025

President Donald Trump threatened 100% tariffs on some drug imports.
Via Investor's Business Daily · September 26, 2025

Both companies have made significant progress in recent years.
Via The Motley Fool · September 4, 2025

Via Benzinga · September 3, 2025

FDA approves Tonmya, a non-opioid fibromyalgia therapy from Tonix, with strong trial data and U.S. launch expected in late 2025.
Via Benzinga · August 18, 2025


